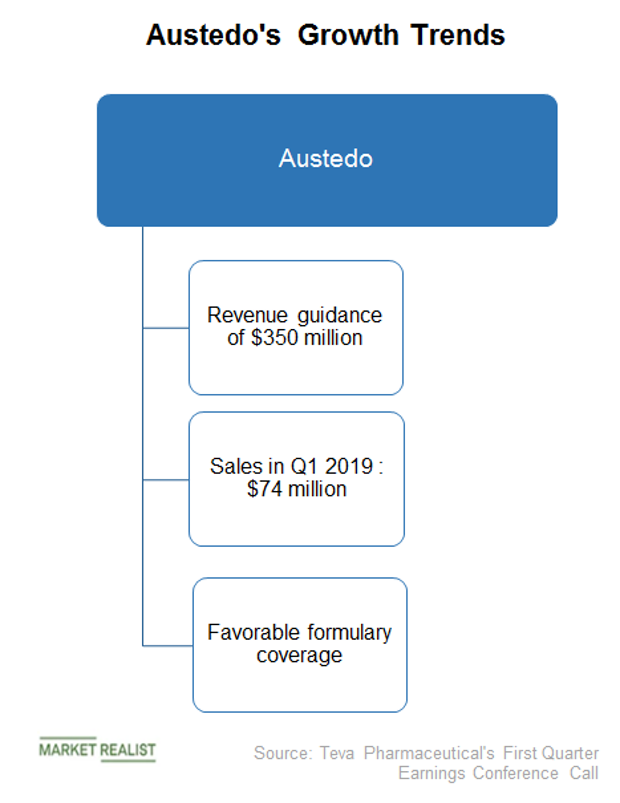
Teva announces FDA approval of Austedo tablets for chorea associated with huntington's disease - Pharma Advancement
Austedo (deutetrabenazine) for the Treatment of Chorea Associated with Huntington's Disease - Clinical Trials Arena

Teva launches first DTC for Austedo into competitive tardive dyskinesia 2-drug market | Fierce Pharma

Austedo for Huntington's disease: Teva Pharmaceuticals has found a nifty way to keep drugs in your body for longer — Quartz

Teva Announces Post Hoc Analysis of Long-Term Data Examining Treatment with AUSTEDO® (deutetrabenazine) Tablets in Adult Patients with Tardive Dyskinesia